To join eCPT℠, reach out to your CPT℠ sales representative.
CPT℠ takes pride in providing accurate and reliable study data in a timely manner.
To help you easily access records of your organization’s testing results, we created eCPT℠, a portal that allows users to securely view these records whenever needed.
Learn more about our portal by contacting your Account Manager or Client Services (clientservices@cptclabs.com).
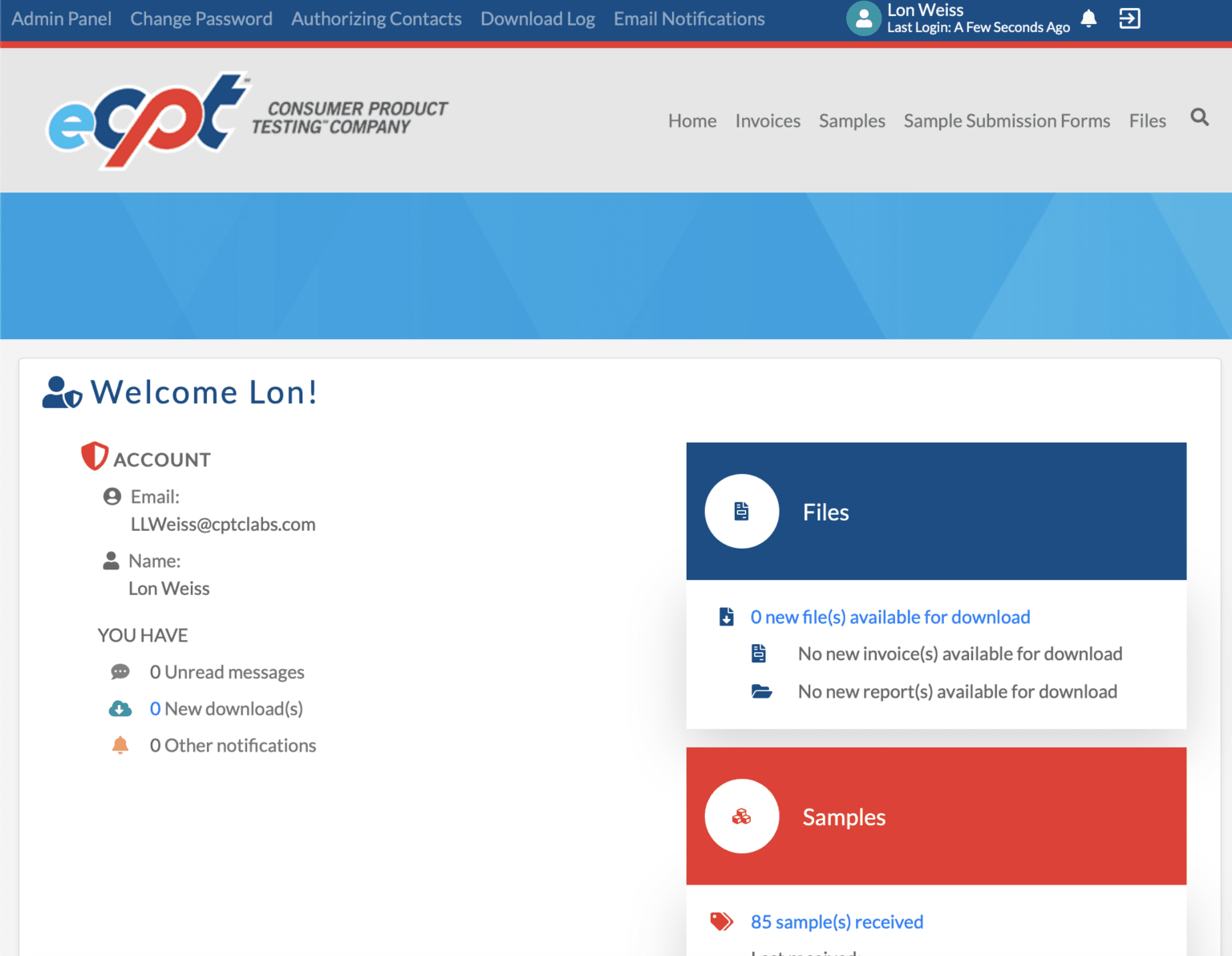
To join eCPT℠, reach out to your CPT℠ sales representative.
Corporate Headquarters and Labs
70 New Dutch Ln
Fairfield, NJ 07004
973-576-5957
Satellite Clinical Lab Location
304 Wootton St.
Boonton, NJ 07005
FDA
Registration FEI # 1000151293 as a GMP/GLP/GCP Drug Establishment and Cosmetic Facility.
DEA
Registration# RC0199744 (Analytical Lab)
Schedule I-V license
Remember, Schedules I-II require DEA Form 222
Registration# RC0171568 (Researcher)
US EPA/NJ DEP
Registration# NJD982726648
ISO/IEC 17025:2017 Accreditation # 80071
