
What to Look for When Hiring a Testing Lab
Testing for product safety and claim support is crucial in the highly competitive, possibly soon-to-be-regulated personal care market. If you are just getting started, or

When it comes to launching a new OTC Drug product in the U.S., CPT℠ Labs can provide expert stability testing and consulting assistance nearly every step of the way.
This includes early-stage consulting assistance in assuring that your new product complies with the correct OTC Drug Monograph; raw material and container/closure system testing; In-process, Product Release and Stability testing; Method Development and Method Validation services; Process Validation and Cleaning Validation testing; OTC Monograph efficacy testing (SPF, for example).
You can rely upon our expert capabilities to assist you in bringing your new OTC Drug product to market in the most efficient and compliant manner.
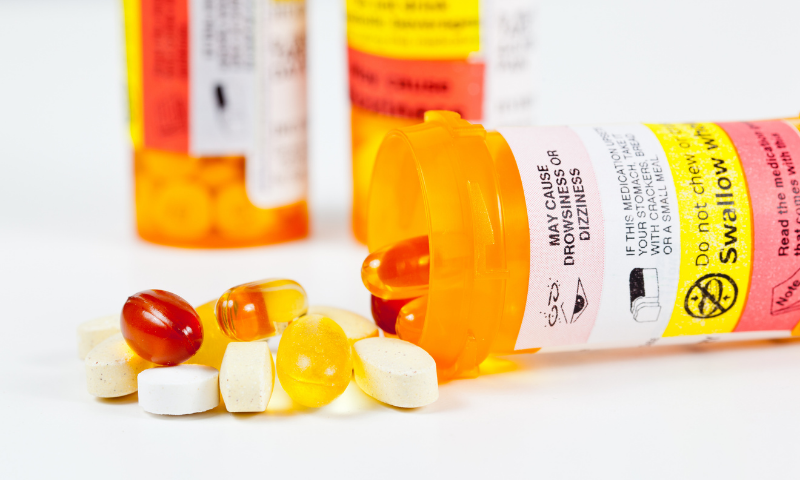
Virtually all Over-the-Counter (OTC) and Prescription (Rx) drug products distributed within the USA are required to include an expiration date on their labeling. Expiration dates must be supported by stability testing which has been conducted in accordance with current FDA and ICH Guidelines and cGMP requirements.
Each strength of a drug product and each primary package and closure system in which it is distributed must be supported by stability testing. This requirement may be found in the Code of Federal Regulations, 21 CFR Part 211.137 – “Expiration Dating” and Part 211.166 – “Stability Testing”.
Stability studies are required to be conducted on drug products under the conditions of storage appearing on the product label, including any other conditions that the drug product might be exposed to during manufacturing, storage and shipping activities.
As a result, there are a number of stability conditions that are commonly employed to determine the stability of a drug product in its marketed package(s). Examples of the most common types of stability studies are as follows:
A stability study conducted under controlled temperature and humidity approximating conditions of long-term storage.
For drug products distributed within the USA, long-term stability storage conditions are 25 +/- 2oC with 60 +/- 5% RH.
A stability study conducted under exaggerated conditions in an attempt to accelerate the aging process, i.e., the rate of chemical degradation and/or physical change.
For drug products distributed within the USA, virtually all accelerated stability studies are conducted under storage conditions of 40 +/- 2o C with 75 +/- 5% RH.
A stability study that is conducted under conditions that are between long-term and accelerated storage conditions.
For drug products sold within the USA, intermediate conditions are 30 +/-2oC with 65 +/- 5% RH.
Samples stored under intermediate conditions are typically only tested if a failure is encountered during the testing of samples stored under accelerated storage conditions.
A study that is conducted at 5oC (without humidity control) to determine the effect that freezing and subsequent thawing has upon the stability of a product.
Such studies may be conducted with single or multiple freeze/thaw cycles, with three (3) cycles being the most commonly used.
A study that is conducted to determine the effect that either whole or ultraviolet light has upon the stability of a product.
This is not required for drug products packaged in opaque container systems. (Refer to ICH Guideline Q1B “Stability Testing: Photostability Testing of New Drug Substances and Products”.)
A stability study that is conducted under long-term conditions to assess the stability of a product batch in its bulk container.
This applies to situations where a drug product may be stored in a bulk container for a period of time prior to its placement into end-user packaging.
A study that is conducted to determine the period of time that a product remains suitable for use after its container system has been opened.
There currently is no requirement for the determination of PAO for any product distributed within the USA. However, the EU requires the determination of PAO and the displaying of the PAO on the labeling of cosmetic products having an expiration date of 30 months durability or greater. Products with less than 30 months durability require a best used by date.
Although not required in the USA, some manufacturers have started to implement the practice of determining the PAO for products that they distribute within the USA.
This may be driven by savvy consumers who may be familiar with and prefer the EU practice of displaying PAO on product labeling, as they are beginning to make their preferences known to US manufacturers.
FDA requires that the expiration date of each OTC drug product be supported by Real-Time (RT) stability studies. That being said, FDA understands that drug owners would face undue hardship if they were required to wait the full two years it would take to generate RT stability data before launching a new OTC drug product with a 2-year expiration date.
As a result, FDA allows for the extrapolation of accelerated stability data for determining expiry dating for the initial launch. However, FDA expects that the same batch that was used for the accelerated study also undergo concurrent RT stability testing. Accelerated stability studies alone cannot be used to support the expiration dating of OTC drug products. The results of accelerated studies must be confirmed by conducting RT stability studies.
As stated in the previous section, the results of accelerated stability studies may be used to approximate the shelf life of a new OTC drug product for launch.
Historically, the FDA has permitted three (3) months of acceptable accelerated stability test results to support a 2-year expiration date for a product launch. However, in recent years, FDA has tightened up its stability requirements for Rx drugs, now requiring six (6) months of accelerated stability and one (1) year of RT stability data at the time of submission.
Based on this data, the FDA now commonly grants only an 18-month (sometimes less) expiration date. Since OTC drug policy eventually tends to follow along the lines of Rx drug policy, it is prudent for the launch of new OTC drug products, that six (6) months of accelerated stability testing now be conducted to support a 2-year expiry date for the launch.
It should also be noted that anything less than three (3) months of acceptable accelerated stability data cannot be routinely used to support any expiry date.
There are only two (2) ways to support a 3-year expiry date for an OTC drug product:
Extending the accelerated storage time or storing under harsher conditions will not routinely support a 3-year expiration date.
Please Note: CPT℠ only performs testing for companies. We do not perform product testing for the general public.

Testing for product safety and claim support is crucial in the highly competitive, possibly soon-to-be-regulated personal care market. If you are just getting started, or

Understanding method validation vs. verification is vital when trying to launch your next regulated product. When testing regulated products such as Pharmaceuticals (Prescription and Over-the-counter),

The Food & Drug Administration (FDA) has a standardized set of principles that ensure the safety and consistency of pharmaceutical and cosmetic products. Being compliant
Stability studies are required to be conducted on drug products under the conditions of storage appearing on the product label, including any other conditions that the drug product might be exposed to during manufacturing, storage and shipping activities.
As a result, there are a number of stability conditions that are commonly employed to determine the stability of a drug product in its marketed package(s). Examples of the most common types of stability studies are as follows:
A stability study conducted under controlled temperature and humidity approximating conditions of long-term storage.
For drug products distributed within the USA, long-term stability storage conditions are 25 +/- 2oC with 60 +/- 5% RH.
A stability study conducted under exaggerated conditions in an attempt to accelerate the aging process, i.e., the rate of chemical degradation and/or physical change.
For drug products distributed within the USA, virtually all accelerated stability studies are conducted under storage conditions of 40 +/- 2o C with 75 +/- 5% RH.
A stability study that is conducted under conditions that are between long-term and accelerated storage conditions.
For drug products sold within the USA, intermediate conditions are 30 +/-2oC with 65 +/- 5% RH.
Samples stored under intermediate conditions are typically only tested if a failure is encountered during the testing of samples stored under accelerated storage conditions.
A study that is conducted at 5oC (without humidity control) to determine the effect that freezing and subsequent thawing has upon the stability of a product.
Such studies may be conducted with single or multiple freeze/thaw cycles, with three (3) cycles being the most commonly used.
A study that is conducted to determine the effect that either whole or ultraviolet light has upon the stability of a product.
This is not required for drug products packaged in opaque container systems. (Refer to ICH Guideline Q1B “Stability Testing: Photostability Testing of New Drug Substances and Products”.)
A stability study that is conducted under long-term conditions to assess the stability of a product batch in its bulk container.
This applies to situations where a drug product may be stored in a bulk container for a period of time prior to its placement into end-user packaging.
A study that is conducted to determine the period of time that a product remains suitable for use after its container system has been opened.
There currently is no requirement for the determination of PAO for any product distributed within the USA. However, the EU requires the determination of PAO and the displaying of the PAO on the labeling of cosmetic products having an expiration date of 30 months durability or greater. Products with less than 30 months durability require a best used by date.
Although not required in the USA, some manufacturers have started to implement the practice of determining the PAO for products that they distribute within the USA.
This may be driven by savvy consumers who may be familiar with and prefer the EU practice of displaying PAO on product labeling, as they are beginning to make their preferences known to US manufacturers.
FDA requires that the expiration date of each OTC drug product be supported by Real-Time (RT) stability studies. That being said, FDA understands that drug owners would face undue hardship if they were required to wait the full two years it would take to generate RT stability data before launching a new OTC drug product with a 2-year expiration date.
As a result, FDA allows for the extrapolation of accelerated stability data for determining expiry dating for the initial launch. However, FDA expects that the same batch that was used for the accelerated study also undergo concurrent RT stability testing. Accelerated stability studies alone cannot be used to support the expiration dating of OTC drug products. The results of accelerated studies must be confirmed by conducting RT stability studies.
As stated in the previous section, the results of accelerated stability studies may be used to approximate the shelf life of a new OTC drug product for launch.
Historically, the FDA has permitted three (3) months of acceptable accelerated stability test results to support a 2-year expiration date for a product launch. However, in recent years, FDA has tightened up its stability requirements for Rx drugs, now requiring six (6) months of accelerated stability and one (1) year of RT stability data at the time of submission.
Based on this data, the FDA now commonly grants only an 18-month (sometimes less) expiration date. Since OTC drug policy eventually tends to follow along the lines of Rx drug policy, it is prudent for the launch of new OTC drug products, that six (6) months of accelerated stability testing now be conducted to support a 2-year expiry date for the launch.
It should also be noted that anything less than three (3) months of acceptable accelerated stability data cannot be routinely used to support any expiry date.
There are only two (2) ways to support a 3-year expiry date for an OTC drug product:
Extending the accelerated storage time or storing under harsher conditions will not routinely support a 3-year expiration date.
Corporate Headquarters and Labs
70 New Dutch Ln
Fairfield, NJ 07004
973-576-5957
Satellite Clinical Lab Location
304 Wootton St.
Boonton, NJ 07005
FDA
Registration# 1000151293
CPT℠ is registered with FDA (FEI # 1000151293) as a Drug Establishment (GMP/GLP/GCP) and a Cosmetic Facility.
DEA
Registration# RC0199744 (Analytical Lab)
Schedule I-V license
Remember, Schedules I-II require DEA Form 222
Registration# RC0171568 (Researcher)
US EPA/NJ DEP
Registration# NJD982726648