USP <81> Antibiotic Potency Testing Methods
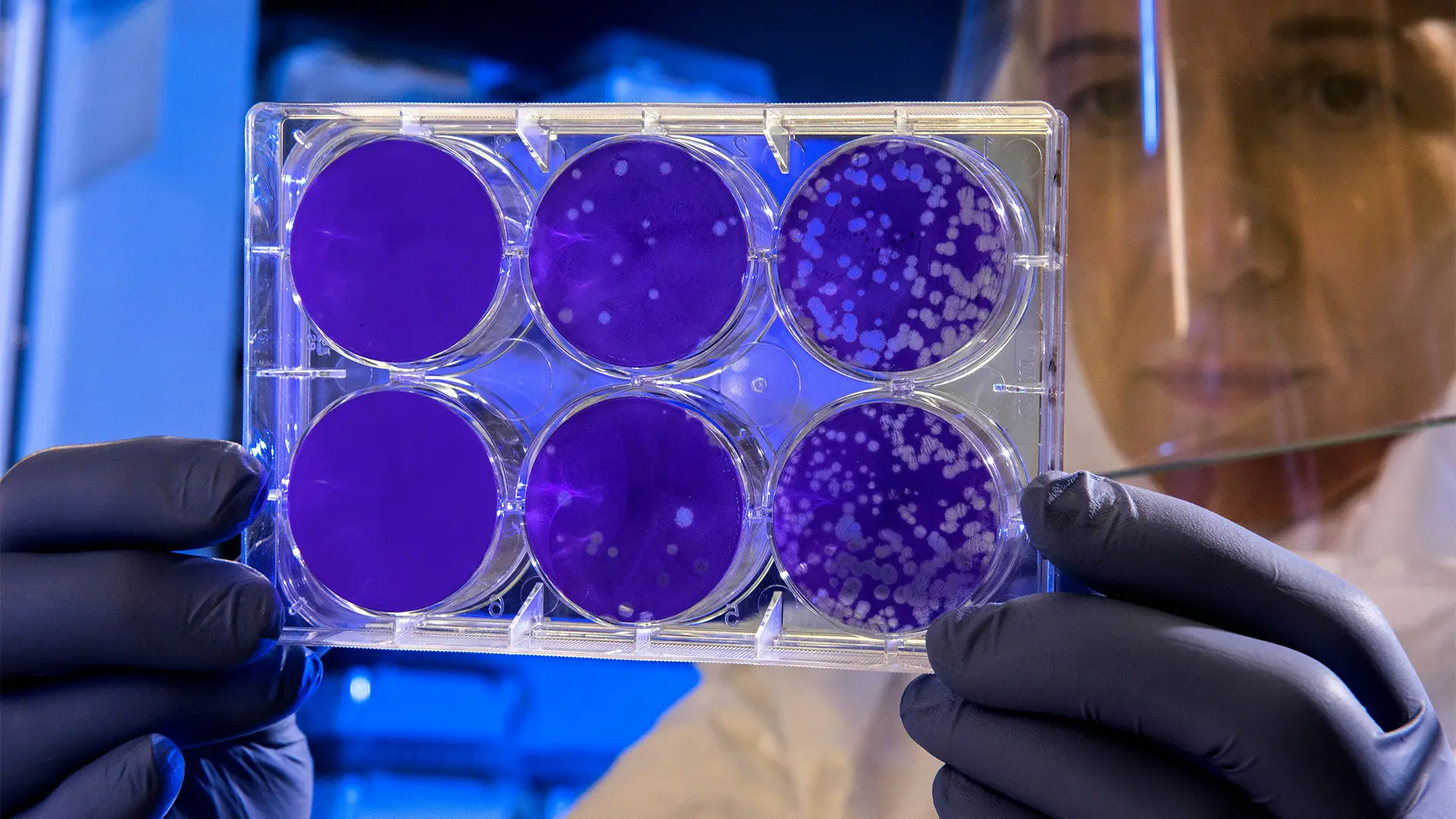
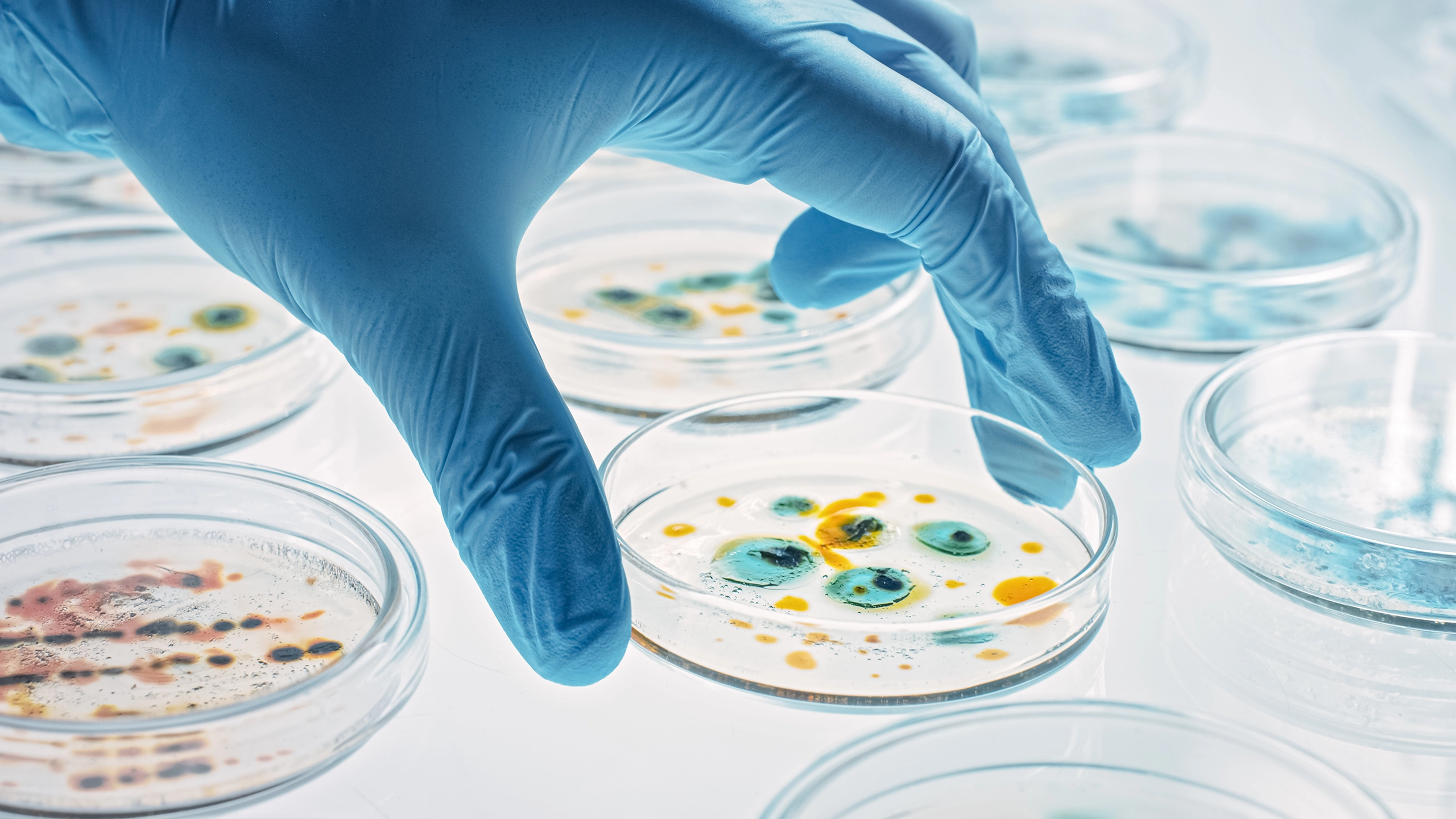
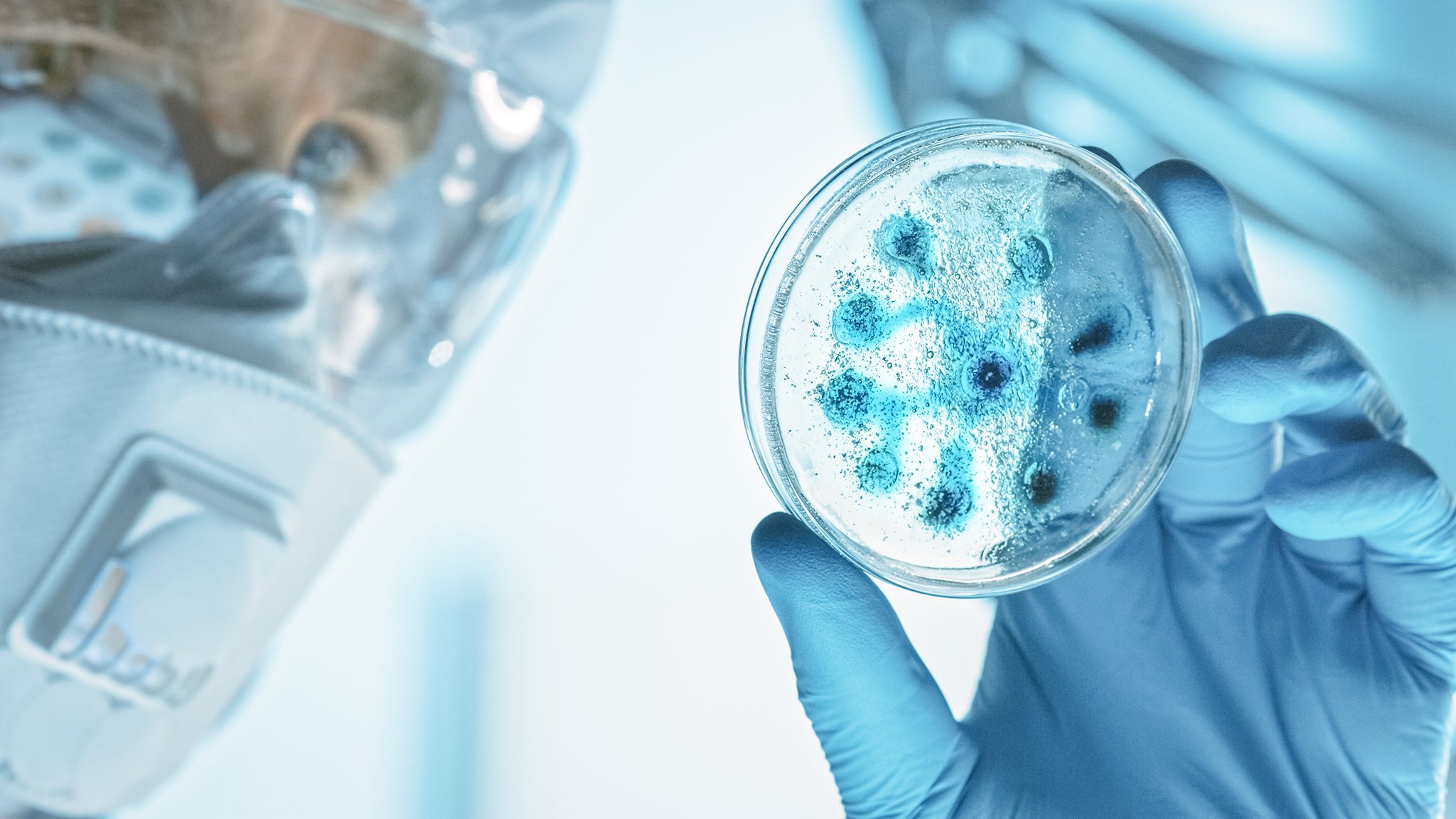
Antibiotic Potency Testing in Pharmaceuticals Antibiotic potency testing plays a pivotal role in the pharmaceutical industry, ensuring that antibiotic products are both effective and safe for consumer use. As antibiotics are critical tools in combating bacterial infections, maintaining their efficacy is essential to public health. Potency testing verifies that these